56+ how to calculate screen failure rate in clinical trials
Web Standardized screen failure data in clinical trials is hard to find and results across different types of studies are rarely published. Web blueReliability engineering is a branch of statistics and probability that is used to calculate the failure rate of machines and parts.

Screen Failure Rates Vs Number Of Patients Recruited Covance Download Scientific Diagram
Average Recruitment Rate ARR of the study is when you calculate all recruited.

. For every Yaw in that application and there are many there is a power you may not have tapped. Web These ubiquitous rates make sense to us because we can intuitively understand from them that sites may be screening and randomizing patients in a way that is consistent with our. Failure rate 1MTBF RT where R is the number of failures.
Web This study examined two episodic memory measures the Repeatable Battery for the Assessment of Neuropsychological Status RBANS and the Cogstate International. Web If the MTBF is known one can calculate the failure rate as the inverse of the MTBF. The formula for failure rate is.
A reliability engineer uses historic records of. Web In terms of screen failure the development of symptomatic treatment in mild to moderate AD has been associated with rates ranging between 15-35. Web We developed automated classification models to identify eligible patients for prehospital clinical trials using EMS clinical notes and compared model performance to.
One review of clinical trials for. Web Here we examine screen failure rates by race in patients screened for participation in clinical trials of acute myeloid leukemia AML. Web In the world of trial management Excel makes up 78 of the atmosphere.
Web The main reason for screen failures among all prostate cancer clinical trials was ineligibility. Among 14 phase 3 trials in kidney cancer 5 reported rates and the. Both of them have pros and cons.
Web There are two main ways to do the calculation. Web The recruitment rate for clinical trials is calculated by multiplying the number of sites and randomized patients per site by the number of months of recruitment time.
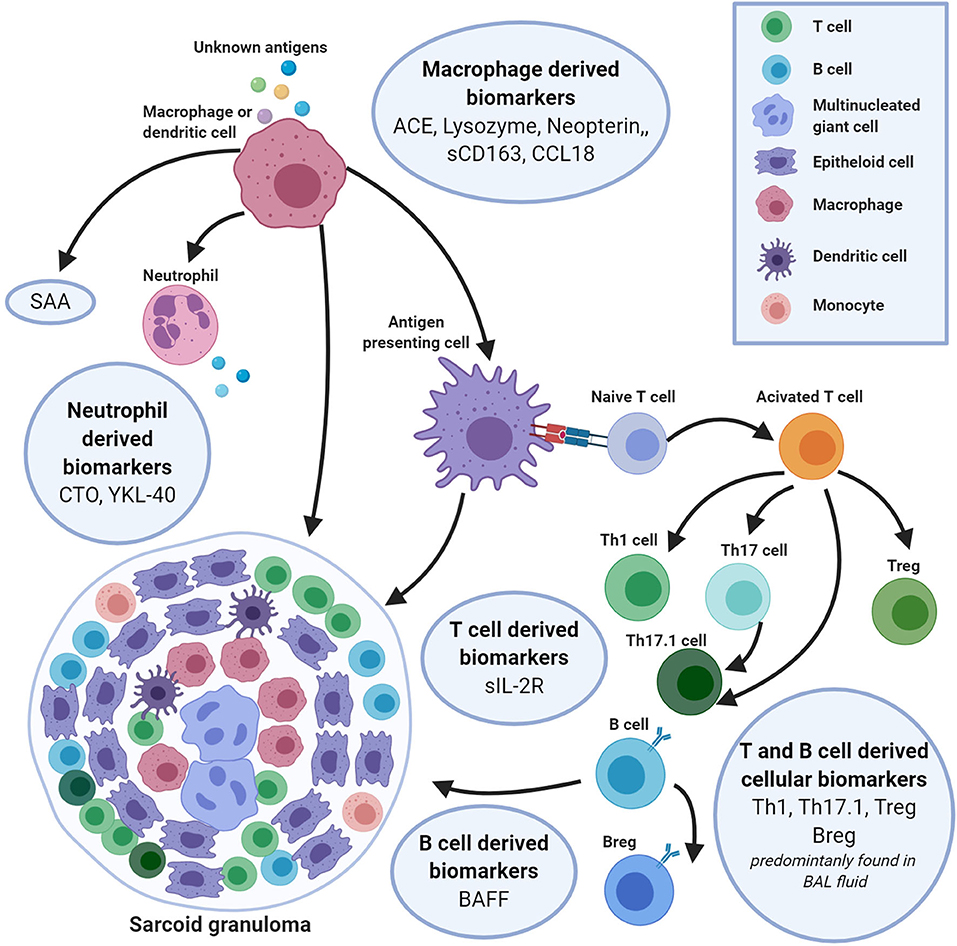
Frontiers Biomarkers In The Diagnosis And Prognosis Of Sarcoidosis Current Use And Future Prospects

Recent Advances In Anti Cancer Protein Peptide Delivery Bioconjugate Chemistry

Fast Track Cities 2022

2019 Scientific Program 2019 Journal Of Ultrasound In Medicine Wiley Online Library
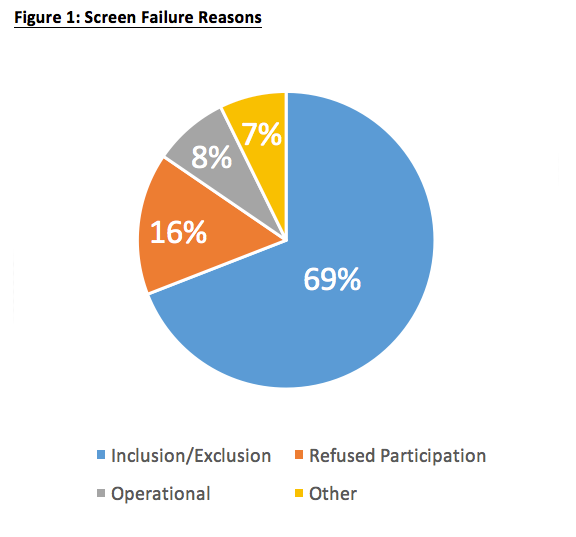
4 Steps To Mitigate Screen Failure Risks

Screen Failure Rates Vs Number Of Patients Recruited Covance Download Scientific Diagram
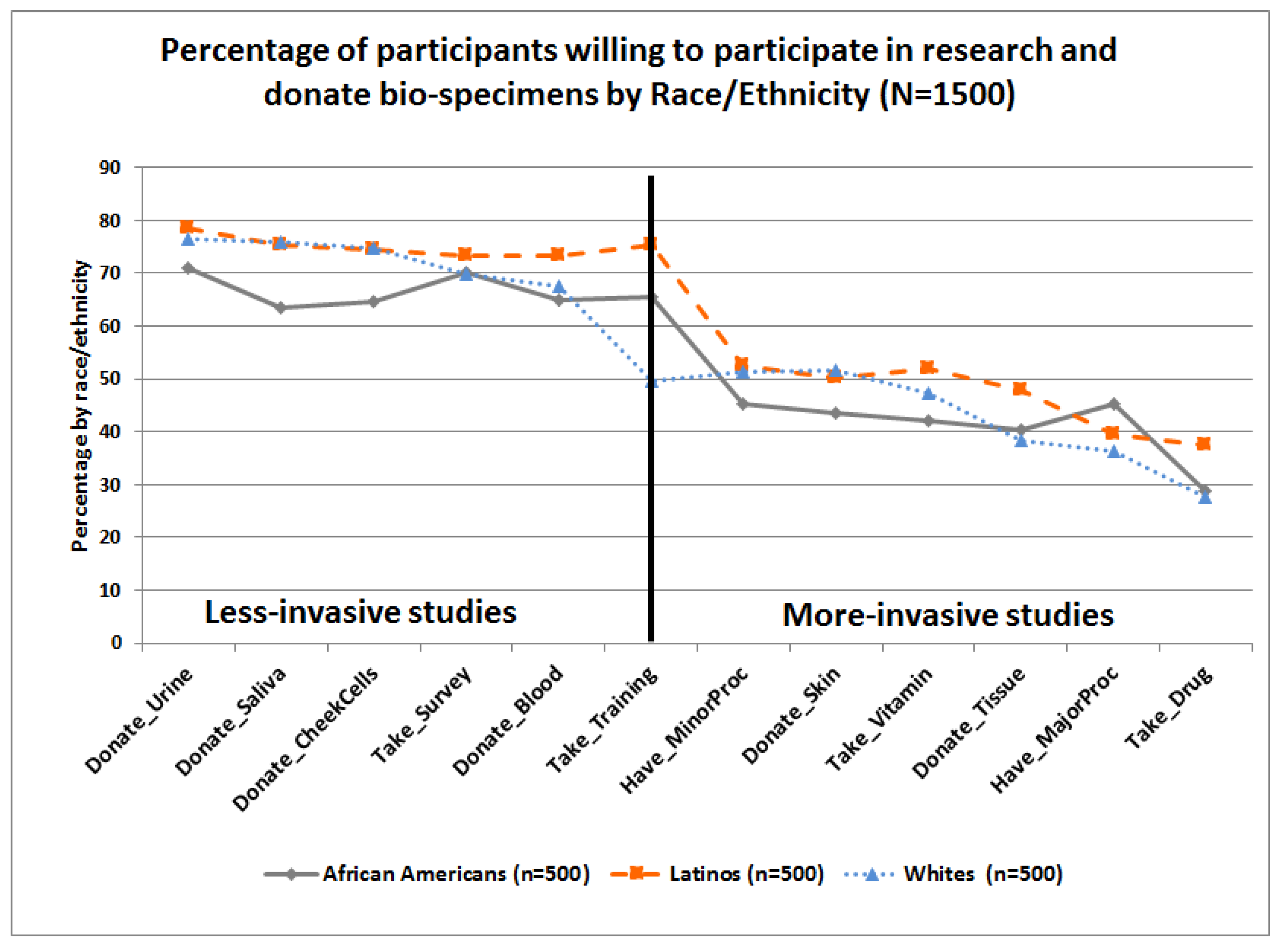
Ijerph Free Full Text Cancer Health Literacy And Willingness To Participate In Cancer Research And Donate Bio Specimens
4 Steps To Mitigate Screen Failure Risks

Fast Track Cities 2022

Screen Failure Rates In Contemporary Randomized Clinical Phase Ii Iii Therapeutic Trials In Genitourinary Malignancies Sciencedirect

On Biostatistics And Clinical Trials Definition Of Screening Failures In Clinical Trials

Speaker Abstracts 2022 Alcoholism Clinical And Experimental Research Wiley Online Library

Recruiting For Studies Ongoing Upcoming Completed National Dental Pbrn

A Multicenter Randomized Study Of Decitabine As Epigenetic Priming With Induction Chemotherapy In Children With Aml Biorxiv

Federal Register Medicare And Medicaid Programs Cy 2018 Home Health Prospective Payment System Rate Update And Proposed Cy 2019 Case Mix Adjustment Methodology Refinements Home Health Value Based Purchasing Model And Home Health

Unique 4 Step Approach Limits High Screen Failure In Alzheimer S Disease Research Part 1 Worldwide Clinical Trials

Optimizing Screen Failures In Clinical Trials Proofpilot